
- #SOP PROJECT MANAGEMENT TEMPLATE SOFTWARE#
- #SOP PROJECT MANAGEMENT TEMPLATE CODE#
Impacts any existing risk control measures.Introduces any new risks and whether those are acceptable.Is a significant change (refer to e.g.If the change does not constitute a bug fix but rather a regular change request, it is evaluated whether the InĬase of a bug fix, the Product Manager directly proceeds with the evaluation as part of the bug fixes In a first step, it is assessed if the change constitutes a bug fix or not.
#SOP PROJECT MANAGEMENT TEMPLATE SOFTWARE#
The Product Manager, together with a Regulatory Affairs Manager and the Head of Software Development, thenĮvaluates the proposed change. ParticipantsĪnyone in the company with a change proposalĭocumented change request (ID per product version) The Product Manager creates a change request ticket in the company’s project management software with aĭescription of the proposed change. They can originate from anywhere inside the company.
Post-Market Surveillance (see process for post-market surveillance). Customer feedback (see process for feedback management). Creation of Change RequestĬhanges proposals can originate from various sources, e.g.: The transitional provision under Article 120 of the MDR with regard to devices covered by certificates Medical Device Coordination Group (MDCG) Document 2020-03: “Guidance on significant changes regarding. Provide it with a supplement to the EU technical documentation assessment certificate.” Shall assess the changes, notify the manufacturer of its decision and, where the changes are approved, Supplement to the EU technical documentation assessment certificate. Notified body shall assess the planned changes and decide whether the planned changes require a newĬonformity assessment in accordance with Article 52 or whether they could be addressed by means of a Inform the notified body which issued the EU technical documentation assessment certificate thereof. Where the manufacturer plans to introduce any of the above-mentioned changes it shall Where such changes could affect the safety and performance of the device or the conditions prescribed for Require approval from the notified body which issued the EU technical documentation assessment certificate Medical Device Regulation, Annex IX Chapter II Section 4.10: “Changes to the approved device shall. Medical Device Directive, Annex II Section 3.4 and Section 4.4. The processing of changes is based on the following regulatory provisions and guidances: Regular Change Requests: All other changes that are not classified as a bug fix. Not 1) Constitute significant changes to the medical device as defined by the criteria outlined in theĬhange assessment list and 2) Introduce new risks or failure modes #SOP PROJECT MANAGEMENT TEMPLATE CODE#
Bug fixes: By definition, bug fixes only constitute minor changes to the software code and complementĮxisting device features instead of introducing new ones or removing existing ones.
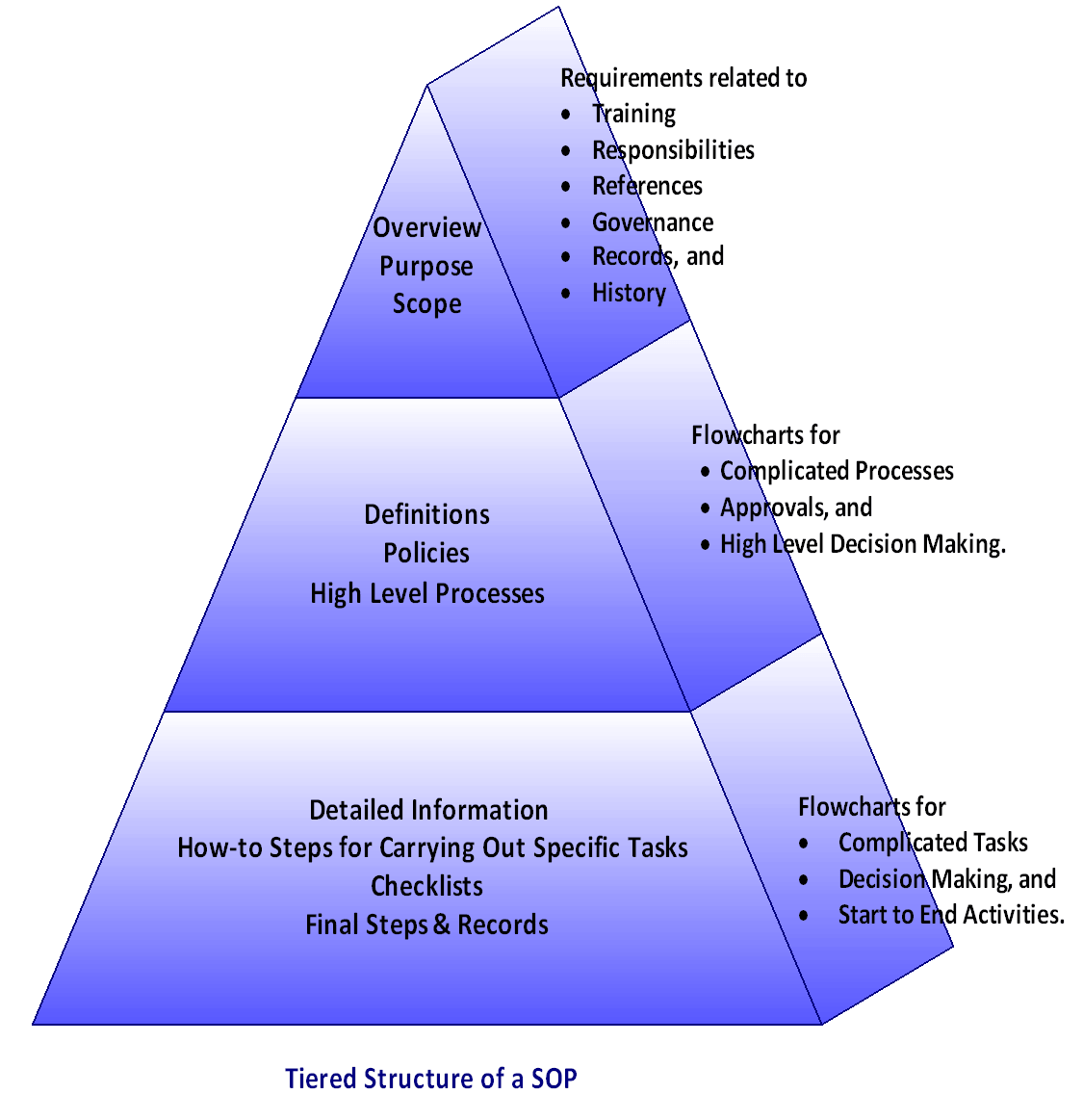
General Considerations Feedback Classificationįeedback is either classified as a bug fix or a regular change request: This SOP describes how we evaluate and make changes to our software after it’s released. Template preview SOP Change Management Classes







 0 kommentar(er)
0 kommentar(er)
